Abstract
Background: Carfilzomib, a second generation selective proteasome inhibitor, is approved in the United States and other countries for the treatment of relapsed or refractory multiple myeloma (RRMM). In the randomized, phase 3 study ENDEAVOR, Kd56 demonstrated clinically and statistically significant improvement compared with Vd in both progression-free survival (PFS) (median 18.7 vs 9.4 months [mos]; hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.44-0.65; 1-sided P <0.0001; Dimopoulos et al, Lancet Oncol . 2016;17:27-38) and overall survival (OS) (median, 47.6 mos vs 40.0 mos; HR, 0.791; 95% CI, 0.648-0.964; 1-sided P=0.0100; Dimopoulos et al, Lancet Oncol . In press). Renal impairment is a common complication of MM and is associated with increased risk of early death. Carfilzomib may be administered in patients (pts) with various degrees of renal impairment including pts on dialysis without starting dose adjustment (Badros et al. Leukemia . 2013;27:1707-14). Here we present a subgroup analysis of the ENDEAVOR trial to evaluate Kd56 and Vd in pts with impaired renal function.
Methods: Adults with RRMM (1-3 prior regimens) and creatinine clearance (CrCL) ≥15 mL/min were eligible for the ENDEAVOR trial. The Cockcroft-Gault formula was used to calculate baseline, and on study, renal function. In the Kd56 arm, pts received carfilzomib (30-min intravenous [IV] infusion) on days (D) 1, 2, 8, 9, 15, and 16 (20 mg/m2 on D1, 2 [cycle 1]; 56 mg/m2 thereafter) and dexamethasone (20 mg) on D1, 2, 8, 9, 15, 16, 22, and 23 of 28-day cycles. Pts in the Vd arm received bortezomib (1.3 mg/m2; IV or subcutaneously) on D1, 4, 8, and 11 and dexamethasone (20 mg) on D1, 2, 4, 5, 8, 9, 11, and 12 of 21-day cycles. Pts were treated until disease progression, physician decision, unacceptable toxicity, withdrawal of consent, or mortality. The primary end point was PFS; secondary end points included OS, overall response rate (ORR), duration of response, rate of grade ≥2 peripheral neuropathy (PN), and safety. The present analyses examined efficacy and safety outcomes in pts grouped according to baseline renal function (CrCL ≥15 to <50, 50 to <80, and ≥80 mL/min). Based on International Myeloma Working Group criteria (Dimopoulos et al, J Clin Oncol . 2016;34:1544-57), a complete renal response was defined as CrCL ≥60 mL/min in any 2 consecutive study visits for pts who had baseline CrCL <50 mL/min
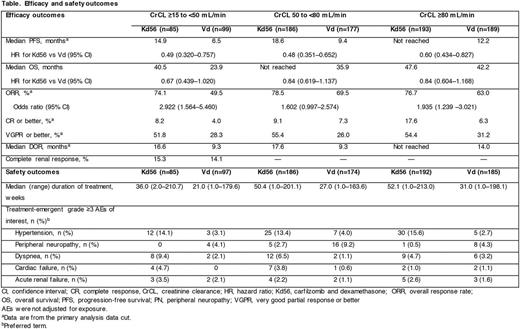
Results: A total of 929 pts were enrolled (CrCL ≥15 to <50 mL/min: Kd56, n=85; Vd, n=99; 50 to <80 mL/min: Kd56, n=186; Vd, n=177; ≥80 mL/min: Kd56, n=193; Vd, n=189). PFS was superior with Kd56 vs Vd within each renal subgroup (CrCL ≥15 to <50 mL/min: median, 14.9 mos vs 6.5 mos [HR, 0.49; 95% CI, 0.320-0.757]; CrCL 50 to <80 mL/min: median, 18.6 mos vs 9.4 mos [HR, 0.48; 95% CI, 0.351-0.652]; CrCL ≥80 mL/min: median, not reached vs 12.2 mos [HR, 0.60; 95% CI, 0.434-0.827]) (Table). OS was longer with Kd56 vs Vd for patients with baseline CrCL ≥15 to <50 mL/min (median, 40.5 mos vs 23.9 mos [HR, 0.67; 95% CI, 0.439-1.020]), CrCL 50 to <80 mL/min (median, not reached vs 35.9 mos [HR, 0.84; 95% CI, 0.619-1.137]) and CrCL ≥80 mL/min (median, 47.6 mos vs 42.2 mos [HR, 0.84; 95% CI, 0.604-1.168]). ORRs were higher in the Kd arm compared with the Vd arm in each renal subgroup (Table). In pts with CrCL ≥15 to <50 mL/min, complete renal response rates were 15.3% for Kd56 and 14.1% for Vd. The median treatment duration was longer with Kd56 than with Vd (median [range], 48 [1-213] vs 27 [1-198] weeks) in the overall population and across renal subgroups (Table). Grade ≥3 AE rates for Kd56 vs Vd were 87.1% vs 79.4% (CrCL ≥15 to <50 mL/min), 83.9% vs 71.8% (CrCL 50 to <80 mL/min), and 76.6% vs 65.9% (CrCL ≥80 mL/min). Rates of grade ≥3 ARF, hypertension, cardiac failure, and dyspnea were higher with Kd56 vs Vd across renal subgroups (Table). Grade ≥3 PN rates were lower in the Kd56 arm compared with Vd arm across renal subgroups (Table). AEs were not adjusted for exposure.
Conclusions: To our knowledge, ENDEAVOR is the largest randomized trial in RRMM to have included pts with severe renal impairment. Superior efficacy for Kd56 vs Vd was observed across all renal subgroups. The safety profile was consistent with the findings from the previous interim analysis. Overall, these data suggest that Kd56 has a favorable benefit-risk profile and should be considered as the new standard of care in pts with RRMM, regardless of baseline renal function.
Dimopoulos: Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology: Consultancy, Honoraria, Other: Advisory Committee: Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology; Genesis Pharma: Research Funding; Novartis: Consultancy, Honoraria. Siegel: Celgene, Takeda, Amgen Inc, Novartis and BMS: Consultancy, Speakers Bureau; Merck: Consultancy. White: Bristol-Myers Squibb: Consultancy, Honoraria; Amgen, Celgene, Janssen, Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Boccia: Pfizer Inc., Sandoz: Other: Advisory Board. Yang: Amgen Inc.: Employment, Equity Ownership. Kimball: Amgen: Employment, Equity Ownership. Iskander: Amgen: Employment, Equity Ownership. Mezzi: Amgen, Inc.: Employment, Equity Ownership. Ludwig: Janssen-Cilag: Consultancy, Speakers Bureau; Bristol-Meyers: Speakers Bureau; AMGEN: Consultancy, Research Funding, Speakers Bureau; Celgene: Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; Takeda: Research Funding, Speakers Bureau. Niesvizky: Amgen: Consultancy; Celgene: Consultancy; BMS: Consultancy; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.